Piano-stool metal complexes as inhibitors of amyloid-β aggregation in vitro and in vivo
Gloria Vigueras, Raimon Sabate, Leoní A. Barrios, Ana B. Caballero, Samanta Hernández-García, Pau Bayón, Fernando Gandía-Herrero, José Ruiz and Patrick Gamez
Inorg Chem. Front., 2024, 11, 6089-6102.
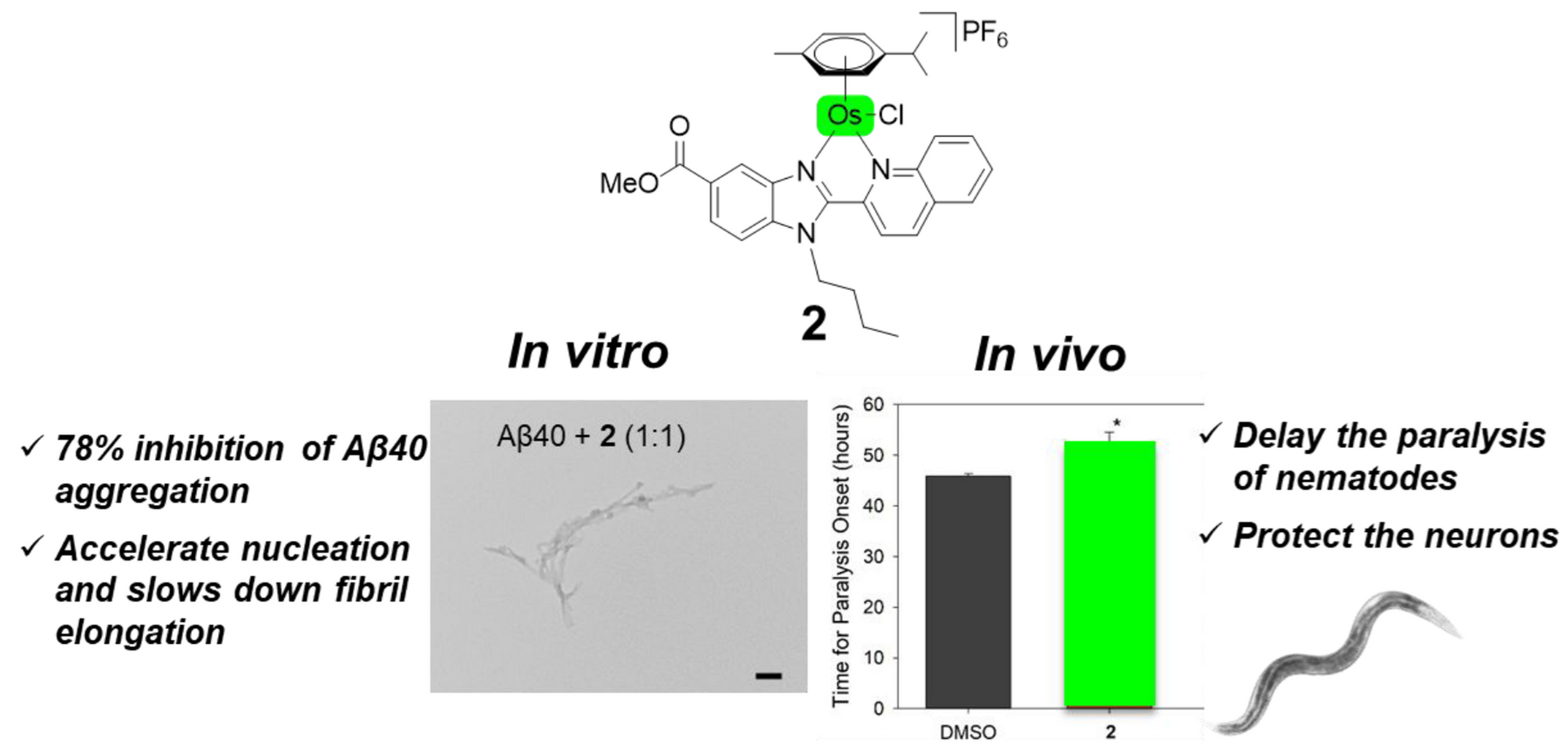
Alzheimer's disease (AD) is the most common form of dementia worldwide. There is currently no cure for this neurodegenerative disorder, and the available therapies only temporarily lessen some symptoms rather than stopping the disease's progression. One of the pathological hallmarks in AD brains is the formation of amyloid-β plaques. Transition-metal complexes have aroused great interest as potential chemical modulators of Aβ aggregation thanks to intrinsic features such as the metal oxidation state or the coordination geometry. Four related piano-stool complexes with different metal ions, namely Ru(II), Os(II), Ir(III) and Rh(III), were prepared and their inhibition properties of Aβ aggregation were investigated. It was found that all of them favour an alternative folding pathway, impeding the formation of mature fibres. Moreover, the metal centre seems to play a crucial role in their inhibiting activities. Ru(II) (1), Ir(III) (3) and Rh(III) (4) compounds were remarkable inhibitors of Aβ aggregation in vitro, most particularly 4, and they appear to share the inhibitory mechanism. However Os(II) complex 2 acts differently on the Aβ aggregation process. In vivo studies using a Caenorhabditis elegans animal model of AD revealed the potential of complexes 2 and 4, with 2 exhibiting better inhibition potential than 4, thus illustrating the possible occurrence of additional variables to the aggregation process when moving from in vitro experiments to a living organism. To the best of our knowledge, 2 is the first osmium complex reported as an effective inhibitor of amyloid-β aggregation both in vitro and in vivo.